What is artificial transmutation? Describe the experiment that led to the discovery of artificial transmutation? Why only high energy α -Particles were used in the discovery of artificial transmutation and neutron?
Artificial Transmutation: The process of converting a stable nucleus into other stable nucleus by bombarding with high energetic particles like α -particles is called artificial transmutation of elements.
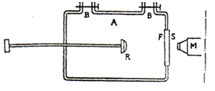
Experiment: Rutherford allowed α -particles to pass through different gases and studied the different processes that may happen.
The apparatus consists of a thick glass chamber A provided with an adjustable rod, carrying a radioactive substance R (radium C). The side of the glass tube facing R is covered by metal plate with a central hole which is closed by a thin silver foil. A screen S coated with a fluorescent material like zinc sulphide is arranged in front of the silver foil and the scintillations produced on it can be observed through the microscope M.
The radioactive substance emits α -particles whose range in air is about 8cm. when the glass tube is filled with nitrogen gas scintillations are observed even when R is at a distance of 40cm from the foil. These particles producing scintillations cannot be α -particles as they cannot have such a long range.
Further analysis proved that each of these particles had a mass nearly equal to that of hydrogen atom and carried a positive charge equal to that of an electron. The new particles were named as protons.
When nitrogen nucleus hit by an α (2He4 ) -particle, it disintegrates into oxygen nucle4us and a proton 1H1 . The nuclear reaction can be represented as
7N14+2He4→8O17+1H1
The light elements from boron to potassium, with the exception of carbon and oxygen will be transmuted by bombarding with α -particles ( 2He4 ) . One such study led to the discovery of neutron.