ELECTROCHEMICAL THERMODYNAMICS
Notation:
To avoid repetitive drawing of complicated cells diagram, a common notation has been adopted for cells. All electrical contacts between half-cells are assumed changes of phase are denoted by |, a salt bridge is shown as || and a junction between two different solutions (a liquid junction in a glass frit) by. If there are multiple species in the same phase these are separated by commas (,). The cell is shown starting at the left-hand electrode and moving to the right-hand electrode through the solution. Examples of cells under standard conditions are:
Pt|H2(g, p=1 atm)|H+(aq, a=1)||Cl-(aq, a=1)|AgCl(s)|Ag(s)
Pt|Fe3+(aq, a=1), Fe2+(aq, a=1)||Zn2+(aq, a=1)|Zn(s)
Formal cell reaction:
The overall formal cell reaction is obtained by writing both half-cell reactions as reductions, e.g.
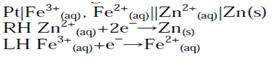
The number of electrons in each equation is then made equal (if necessary):
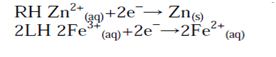
As with the half-cell potentials, the LH reaction is subtracted from the RH to give the formal cell reaction. Minus the LH reaction is equivalent to reversing the reaction (writing it as an oxidation):
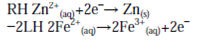
which is followed by combining species on the same side of the reaction arrows together and cancelling those which appear on both sides to give:
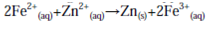
which is the formal cell reaction.