Selection Rule for the Vibrational Spectroscopy:
The selection rule for a spectroscopy refers to the condition that tells us about the transitions that are possible (or allowed) amongst the quantised energy levels. The selection rule for the vibrational spectroscopy is, ?v = ±1, ±2, ±3...etc.. This means that the molecule may go over from any vibrational level to any other level. This in turn means that the spectrum would be quite complex. However the actual IR spectrum for a diatomic molecule is not so complex. The pure vibration spectrum of a diatomic molecule consists of mainly three signals: an intense signal for the fundamental vibration, which arises from the transitions from ground state (v = 0) to the first (v = 1) excited vibrational level. The second is a weak signal for its first overtone that is observed at roughly double the frequency of the fundamental band; it is due to the transitions from ground state (v = 0) to the second (v = 2) excited vibrational stage. The third and the weakest signal is observed at roughly triple the frequency of the fundamental band; it is due to the transitions from ground state (v = 0) to the third (v = 3) excited vibrational level. It is known as the second overtone.
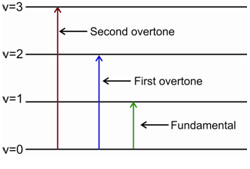
Figure: The transitions for fundamental vibration and the overtones
What will be the nature of spectrum in a polyatomic molecule? Now let us try to extend our understanding of the vibrational behaviour of a diatomic molecule to polyatomic molecules.