VSEPR principles
Stereochemical arrangement becomes a factor whenever an atom is bonded to two or more others. So triatomic species AB2 can be linear (example CO2, ) or bent (example H2O 1, ). It is observed that when a central atom has no nonbonding electrons, the surrounding atoms are generally arranged in a regular way that spaces them as far apart as possible. Though, When nonbonding electron pairs are exist in the valence structure less regular arrangements of bonds are frequently found. The valence shell electron pair repulsion (VSEPR) model is based on the idea that both bonding and nonbonding electron pairs in the valence shell of an atom 'repel' each other. This idea is helpful but can be misleading if taken too literally. Detailed calculations depict that the shape of a molecule is determined by a combination of factors, for which the electrostatic repulsion among electrons is not the most significant. Additionally, the real electron distribution in a molecule is much more consistently spread out than the localized pictures used in VSEPR (1, 2, ...) suggest. It is best to think of 'repulsion' like coming primarily from the exclusion principle, that forces electron pairs to occupy orbitals in different regions of space.
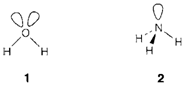
The fundamental principles of the model are as follows.
(i) Valence electron pairs round an atom (whether bonding or nonbonding) adopt a geometry that maximizes the distance among them. The basic geometries generally observed with 2-7 pairs are displayed in Fig. 1.
(ii) Nonbonding electron pairs are closer to the central atom than bonding pairs and have larger repulsions: actually, the order of interactions is
(iii) If double (or triple) bonds are exist the four (or six) electrons involved behave as if they were a single pair, even though they exert more repulsion than do the two electrons of a single bond
(iv) Such as the terminal atoms become more electronegative relative to the central one, bonding electron pairs are drawn away from the central atom and so repel less.