Using VSEPR
It is essential to know the connectivity, i.e. which atoms are bonded together, before applying VSEPR to a complex ion or molecule. With a species of formula AXn this frequently gives no problem, particularly if X is a monovalent atom or group (example H, F, CH3). Sometimes it is not so obvious and a helpful rule (that does not apply to hydrides or organic groups) is that the central atom is generally the least electronegative one. For an instance, in N2O one of the nitrogen atoms is central. Drawing a valence structure includes nonbonding pairs on the central atom then gives the total number of 'pairs' (multiple bonds counted as a single 'pair'). This is sometimes called as
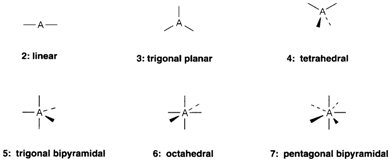
the steric number (SN) of the central atom A, equivalent to the number (n) of bonded atoms, with the number of nonbonding electron pairs. It may usually be assumed that one electron from A is employed in each bond formed to X.
So in SF4 four electrons are used in single S-F bonds, leaving two electrons (that is one pair) nonbonding, thus that the SN=5. In XeOF4 the Xe=O double bond uses two electrons from xenon and again there is one nonbonding pair making SN=6. In the complex ions account must be taken of the charge. So in we can include the charge on the ion and assign eight valence electrons to chlorine. Six are involved in the bonding, as a result there is one nonbonding pair and SN=4.