Steric number 6
The fundamental shape is octahedral and is found with SF6 and PF6-. All positions are equal and with one nonbonding pair AX5 adopts a square pyramidal structure (example BrF5 6, and XeOF4, where repulsion between the double bond and the lone-pair is minimized by putting these trans to each other). While two non-nonbonding pairs are exist they minimize their repulsion (rule ii) by adopting the trans configuration, giving a square planar molecule (example XeF4, 7 and ICl4-).
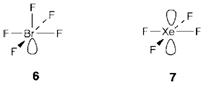
Steric number 7
The only simple instances are the pentagonal bipyramidal IF7 (see diagram. 1. and the ion that is pentagonal planar, that having two lone-pairs occupying the axial positions .