Steric number 5
The general shape adopted by five groups is the trigonal bipyramid, like with PF5. There are now two in equivalent types of position, two axial (top and bottom in Fig. 1) and three equatorial. It shows that the equatorial positions allow more space than axial ones. So bulkier groups (e.g. Cl in PF4Cl) tend to be found in these positions, as do nonbonding pairs when these are present. With consecutively one, two and three nonbonding pairs, molecular shapes are as follows.
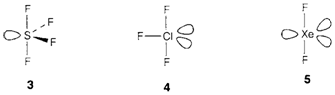
- AX4 is frequently explained as a 'see-saw' with two axial and two equatorial X positions, the former being little bent out of the ideal linear configuration by the lone-pair repulsion. Examples are SF4 (3) and XeO2F2 (where O in preference to F occupies the equatorial position; see the rule no. iii).
- AX3 gives a T-shape, like in ClF3 (4).
- AX2 is linear like the bonded atoms are axial. instances are XeF2 (5) and