Extensions, difficulties and exceptions
One of the difficulties with VSEPR is that its rules show somewhat arbitrary and tough to justify in a rigorous quantum- mechanical formulation. The interpretation of small differences in bond angle is frequently considered to be particularly dubious. Despite this (and of the exceptions noted later) the model is surprisingly helpful. Even though the discussion has concentrated on cases where single atoms are bound to a central one, VSEPR should be capable to predict the geometry around any atom in a complex molecule, in which main-group atoms are involved. (It cannot be usually applied to transition metals;) For instance, in hydroxylamine, H2NOH, the bonds around the nitrogen are pyramidal, those around the oxygen bent as supposed. The model is even helpful in interpreting solid-state structures containing ions like Sn2+ where nonbonding electrons appear to have a stereochemical effect.
One type of exception to VSEPR comes inro existence when apparently nonbonding electrons are actually involved to some extent in bonding. For instance, the geometry around nitrogen is planar when bonded to carbonyl groups in the peptide linkage
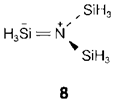
(-NHCO-) in proteins and in trisilylamine, (SiH3)3N (8, only one of three equal resonance structures displayed). In both the cases the 'nonbonding' pair on nitrogen is employed to form partial double bonds. In 8 this needs valence expansion by the silicon and differences with pyramidal trimethylamine (CH3)3N, in which the carbon cannot accommodate extra electrons.
AX5 species with no lone-pairs are some times square pyramidal rather than the normal trigonal pyramid of diagram. 1. Another difficulties come into existence with AX6 where there is one nonbonding pair. This is the case with XeF6, that, as supposed, is not regularly octahedral. Though, A unique shape cannot be determined in the gas phase, as the molecule appears to be highly fluxional and converts quickly among different distorted configurations. By difference, the ions [SeCl6]2- and [TeCl6]2- are regularly octahedral despite having a nonbonding pair. There is no simple description, even though the relatively large size of the chloride ion could be a factor.
Another notable exceptions are some of the group 2 dihalides like BaF2, that in the gas phase are bent and not linear as VSEPR predicts. (In their usual solid-state forms they have distinct structures;.) Two issues that are thought to contribute are
(i) the make use of valence s and d orbitals for bonding (rather than s and p as is normal in later main groups, and
(ii) The probability that core polarization could lower the energy of the bent form.