Three-center bonding
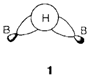
An instance of in which the 2c bonding picture is not possible is in diborane B2H6. The terminal B-H bonds can be explained in simple 2c terms but the number of electrons exist suggests that each bridging hydrogen forms part of a 3c bond involving the two boron atoms. The MO method gives a simple interpretation (1). Four H atoms are disposed approximately tetrahedrally around each boron; this arrangement displays that sp3 hybrids are used. Two such type of hybrids create normal 2c bonds by overlap with the 1s AO on the terminal hydrogens. The others are combined like in 1 to form two 3c bridge bonds. Additionally to B-H overlap there is some direct overlap among the boron hybrids, that provides some B-B bonding as well. The product is known as a three- center two-electron (3c2e) bond. 3c2e bonds with bridging hydrogen take place in other conditions, for instance the normal form of BeH2, that has a polymeric chain structure with all H atoms in bridging positions. Another groups like methyl CH3 can do this, for instance in dimeric aluminum methyl, Al2(CH3)6, that has a structure necessarily identical to B2H6 with CH3 in place of H.
2-
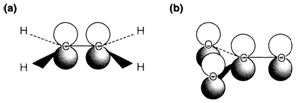
Fig. 2. π bonding MOs in (a) C2H4, (b) CO3.
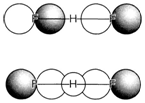
Fig. 3. Occupied MOs in the 3c4e description of [FHF]-.
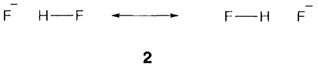
Other type of bridging hydrogen takes palce in the symmetrical ion [FHF]- formed by hydrogen bonding between F- and HF. To know this, first count electrons and orbitals as follows: F 2s AOs contain two electrons each too tightly bound for bonding (as in HF); the 2pπ AOs on each F are too far apart to overlap, so forming nonbonding orbitals holding a total of eight electrons. This leaves four electrons (out of a total valence count of 16) to take MOs created from the two F pσ and the H 1s AO. The two occupied MOs are displayed in Fig. 3. There is a 3c bonding MO in which H 1s is combined with both F pσ Aos and also a nonbonding MO formed from a fluorine combination that has the wrong symmetry to interact with hydrogen. The four electrons within the these MOs give rise to a three-center four- electron (3c4e) bond. Efficiently each F-H bond is only 'half' a covalent bond as in the 3c2e case, but not like that situation there are also two electrons localized on the terminal atoms, giving a negative charge there. The result is equal to the resonance formulation 2.
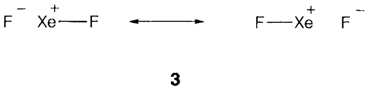
3c4e bonding models are an substitute to the make use of d orbitals in hypervalent compounds with octet expansion. One interpretation of a molecule like XeF2 with five electron pairs around a central atom would use sp3d hybrids, that include d orbitals in the valence shell of Xe. Calculations depict that this picture greatly overestimates the contribution of d orbitals to the bonding. A different approach considers 3c4e bonds that make use of only p orbitals on the central atom. The result related to the resonance picture 3, which needs only eight electrons in Xe valence shell.