Localized and delocalized orbitals
When the molecular orbital (MO) theory is applied to polyatomic molecules alternative explanation are possible, as displayed in Fig. 1 for the linear gas-phase molecule BeH2. There is no reason why an MO must be confined to just two atoms. In diagram. 1a the two orbitals displayed are formed correspondingly from a 2s and a 2p atomic orbital (AO) on beryllium, combined with hydrogen 1s AOs of suitable sign to give a bonding MO. It is also possible to form antibonding combinations (that is not shown). The four valence electrons in the ground state of BeH2 can be considered as occupying the two three-center (3c) or delocalized MOs shown. Bonding stabilization is provided, like in the diatomic case, through a combination of increased electron density in the overlap regions between atoms and a transmission of electrons to the more electronegative hydrogen atoms.
The another picture in diagram 1b is based on sp hybrid orbitals on the central atom, pointing in reverse directions as in the MO description of BH. Each hybrid is combined to create a two-center (2c) or localized MO with the suitable hydrogen AO; again, antibonding MOs can be made but are not displayed. In this explanation of BeH2 two electrons occupy each of the 2c MOs, giving a picture identical to that assumed in VSEPR theory in which two electron pairs around an atom adopted a linear configuration.
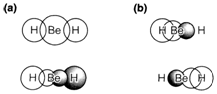
Fig. 1. Bonding MOs for BeH2. (a) 3-center, (b) 2-center representations. In each case both MOs are doubly occupied.
The 3c and 2c bonding descriptions look distinct, but so long as both orbitals are doubly occupied in each case, they are actually equivalent. In the orbital approximation any set of occupied orbitals might be replaced by a linear combination of them with no changing the entire many-electron wavefunction. The two 2c MOs of diagram. 1b can be created by making linear combinations of the 3c MOs in diagram. 1a. and alternatively the 3c MOs could be reconstructed by combining the 2c MOs. The two diagrams depict different ways of 'dissecting' the total electron distribution into contributions from individual pairs, but as electrons are entirely indistinguishable such type of dissections are arbitrary and do not predict any observable variations.
To the polyatomic systems the two MO approaches are localized and delocalized, are helpful in different conditions. When localized explanation is possible, they correspond more closely to the simple chemical pictures of electron-pair bonds provided by the Lewis and VSEPR models. Such type of descriptions is not all the time possible and 3c or other delocalized models give another to the resonance approximately. Delocalized MO theory is also more helpful for interpreting electronic spectra of molecules.