Second period diatomics
In expanding the MO theory to second period elements two additional principles require to be considered. First, only valence-shell orbitals are displayed in MO diagrams, as the inner shells are too tightly bound to be involved in bonding and do not overlap considerably. Second, both 2p and 2s valence orbitals require to be included. The three AOs inside a p shell different only in their direction in space, and in an atom are degenerate (that is have similar energy). Though, in diatomic molecules, they are differentiated by the way they overlap: pσ orbitals point along the direction of the bond and pπ orbitals (of which there are two degenerate and equivalents ones) are perpendicular to it. Every type of orbital can merge to form MOs in similar way as in H2. Two basic rules in the LCAO MO model are significant:
- The no. Of MOs formed is equal to the total number of starting AOs;
- Only AOs of similar symmetry type combine to make MOs.
Figure 3 depicts that the pσ AOs, which point in the direction of each other, overlap more strongly than do pπ MOs, so that both the antibonding destabilization and the bonding stabilization is greater for pσ. The order of MO energies generated from the 2p AO on the two atoms is so:
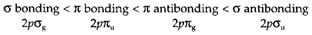
The symmetry designation of every MO is given and it should be noticed that the g or u labeling for π MOs is the opposite of that for σ MOs.
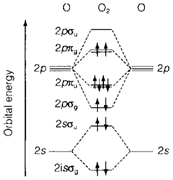
Figure 4 depicts the MO diagram for dioxygen O2. Like in Fig. 2 the energies of 2s and 2p AOs of the separate atoms are displayed and among them the MOs formed from overlap. Lowest in energy are 2sσg and 2sσu, correspondingly the bonding and antibonding combinations of 2s AOs. At the higher energies are MOs resulting from 2p AOs. Figure 4 depicts occupation of MOs in O2 (12 valence electrons). The BO considered as above is two, as supposed from the normal valence structure. Though, the degenerate 2pπg MOs are occupied by two electrons and like in atoms, Hand's first rule depicts that the ground state will be created by putting one electron in each orbital with parallel spin. So O2 is correctly predicted to have two unpaired electrons and as a consequence is paramagnetic.
One of the benefit of MO theory is that similar diagram can be employed for molecules with distinct numbers of electrons. The electron configurations and the BOs for some molecules and ions, that can be derived using Fig. 4. are displayed in Table 1 together with the experimental bond lengths and dissociation energies. Higher predicted BO values correspond to shorter and stronger bonds.
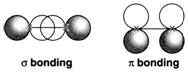
Diagram 3. Bonding MOs formed by σ and π overlap of 2p AOs. Negative regions of the wave function are shaded.
For more sophisticated reasons (example interpretation of molecular spectra) a number of refinements require to make to this simple picture. Particularly, the possibility of overlap between a 2s orbital on one atom and the 2pσ on the other can change the order of MO energies. This does not change the electron configurations of any species displayed in Table 1. but is significant for some molecules like C2 (known at high temperatures, example in flames) and for heteronuclear molecules
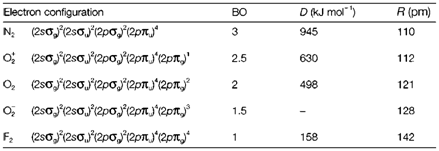
Table 1. Electron configurations, bond orders (BO), dissociation energies (D) and bond lengths (R) for some homonuclear diatomics