HF and BH
HF and BH, the two molecules, show cases where more orbitals are included (see Fig. 2). Fluorine is more electronegative in the HF, and therefore its AOs are lower in the figure than that of H. It may be supposed that the 2s AO on F is quite far apart in energy from the 1s on H to commute extensively. The bonding and antibonding MOs are shaped from amalgamations of H 1s with F 2p. The two pπ AOs on F have no analogous AO on H to interact with (as 1s is of σ symmetry) and so stay nonbonding. The orbital occupancy exposed corresponds to a bond order (BO) of one, because the F 2s orbital is nonbonding too. The bonding orbital is much localized on F and the charge allocation is Hδ+Fδ-.
In BH the electronegativity variations are reversed and bonding orbitals will be more localized on H. Nevertheless, 2s and 2pσ AOs on boron are of equivalent energy and both can add to the bonding. As the integer of MOs produced is identical to the integer of starting AOs, the H 1s and B 2s and 2pσ AOs merge to form three MOs, of which one is bonding, one just about nonbonding and one antibonding. The MOs publicized in Fig. 2 may be understood in terms of sp hybrid AOs produced on boron by mixing 2s with 2pσ. Two such hybrids are produced; one is heading towards hydrogen and one away. The previous hybrid merges with H 1s giving bonding and antibonding amalgamations, while the other doesn't overlie much and is nonbonding. The four valence electrons in BH, hence, make a bonding pair and a nonbonding pair oriented in contradictory directions as expected in the VSEPR model. The bond degree is one, with the charge distribution Bδ+Hδ- as expected on electronegativity grounds.
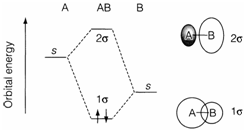
Figure-1 - MO illustration for a heteronuclear molecule with one valences orbital per atom. The form of the MOs is also exposed, with shading representing negative fields of wave-function.
Labelling of MOs in the heteronuclear case, pursue the same σ or π arrangement as for homonuclear diatomics but the subscripts g and u are not specified, as there is no center of inversion symmetry. Dissimilar σ and π MOs are labelled 1, 2, 3,...in order of rising energy. Generally, only valence-shell orbitals are involved but the labelling occasionally involves inner shell orbitals as well. Labelling of the MOs, in Figure-2, chases the usual convention with the MO imitative from the inner shell 1s AO on B or F not involved. Occasionally the position σ* or π* is used to differentiate antibonding MOs from the σ or π bonding MOs.
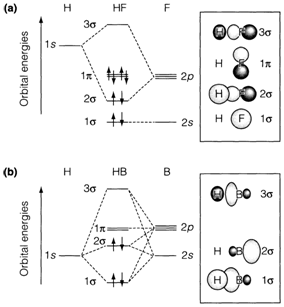
Figure 2 - MO diagrams with the estimated forms of orbitals exposed for (a) HF and (b) BH (negative fields shaded).

Fig. 3. MO diagram for CO, showing the form of the frontier orbitals 3σ and 2π.