Basic principles
In a heteronuclear molecule a bond is created among different atoms, and the most significant difference from the homonuclear case is that molecular orbitals (MOs) are no longer shared equally between atoms. Refer a molecule in which each atom has just one valence atomic orbital (AO): an instance would be gas-phase LiH with 2s on Li and 1s on H. When the MOs are constructed using the LCAO approximation
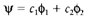
(1)
the coefficients c1 and c2 are no longer equivalent. In LiH the two AOs different greatly in energy, like H has a higher ionization energy and higher electronegativity than Li. If is the AO of lower energy (that is of higher ionization energy or greater electronegativity;), then the bonding MO has c2>c1. The square of every coefficient gives the electron density in the suitable AO, and so the bonding MO has more electron density on the more electronegative atom. By a combination of two effects Bonding is provided: some increase of density among the atoms as in a homonuclear molecule, together with a few electron transfer giving a partially ionic distribution of the form Liδ+ Hδ-. Like the electronegativity variation between atoms increases, therefore does the localization of the MO making the charge distribution more ionic.
Figure 1 depicts the MO diagram suitable to this case. The antibonding MO is also displayed; the electron distribution here is localized in the opposite direction to that in the bonding MO but this orbital is not occupied when only two electrons are exist.
In addition to the providing a explanation of the transition between purely covalent and purely ionic bonding, the model explained above has a consequence which is significant in more complex examples. AOs of extremely dissimilar energy do not mix considerably; the resultants MOs are hardly differ from the AOs themselves and it is a good approximation to neglect their interaction.