Alkene Polymerization
Domestic and industrial plastics are mainly formed through polymerizing alkenes:
The reaction is exothermic and can be started through free radicals (frequently from peroxo compounds), although organometallic catalysts provide more controllable results. Most extensively used are Ziegler-Natta catalysts made through mixing Al2Et6 (in which Et is the ethyl group) along with TiCl4. Solid TiCl3 is created and catalysis takes place at surface Ti-Et groups, for which alkene molecules coordinate and go through insertion into the Ti-R bond. An benefit of these catalysts is that they might create stereoregular polymers in which all the R groups in -C(R)H-CH2- comprise similar stereochemical configuration. This provides stronger materials along with higher melting points than the random stereochemistry resultant from radical polymerization.
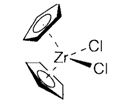
A new generation of catalysts that are based on cyclopentadienyl compounds of early on transition metals like 3, that in the existence of aluminoxane (MeAlO)n creates the active species [(η5-C5H5)2ZrCH3]+.