Batteries:
A battery consists of two or more chemical cells connected in sequence.The combination of materials inside a battery is used for the purpose of converting chemical energy into electrical energy. For understand how a battery works, we have to first discuss the chemical cell.
A chemical cell is composed of two electrodes made of various kinds of metal or metallic compounds that are immersed in an electrolyte solution. A chemical action which result are complicated, and they vary along with the category of material used in cell construction. A few knowledge of the basic action of a simple cell will be helpful in understanding the operation of a chemical cell in common.
Within the cell, electrolyte ionizes to generates positive and negative ions that was shown in the Figure 1, Part A. At the similar time, chemical action causes the atoms inside one of the electrodes to ionize.
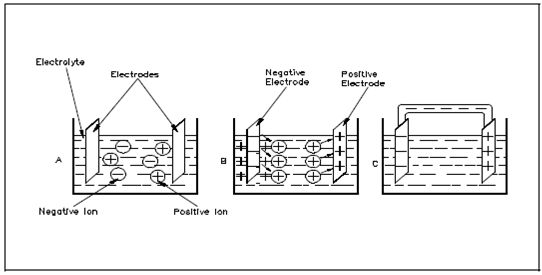
Figure: Basic Chemical Battery
Due to this action, electrons are deposited on the electrode and positive ions from the electrode pass into the electrolyte solution that is discussed in Part B. This causes a negative charge on the electrode and leaves a positive charge within the area near the electrode (Part C).
The positive ions, which were generated through ionization of the electrolyte, are repelled to the other electrode. These ions will combine along with the electrons at this electrode. Since this action causes removal of electrons from the electrode and it becomes positively charged.