Ring flipping of methylcyclohexane:
For instance, methylcyclohexane can have two chair structures in which the methyl group is either on an equatorial bond or on an axial bond.
These are diverse shapes of similar molecule that are inter convertible because of rotation of C-C single bonds (the ring flipping process). The 2 chair structures are conformational isomers however they are not of equivalent stability. The more stable conformation is the one in which the methyl group is within the equatorial position. In this position, the C-C bond linking the methyl group to the ring comprising a torsional angle of 180? with respect to bonds 5-6 and 3-2 in the ring. Though, in the axial position, the C-C bond has a torsional angle of 60? with respect to these similar two bonds.
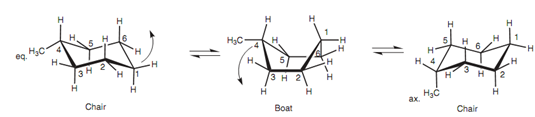
Figure: Ring flipping of methylcyclohexane.