Binary Carbonyls
CO creates binary neutral compounds with several transition metals, and a few anionic and cationic species. Table 1 depicts compounds from the 3d series. Some of these compounds can be get through direct reaction of the metal and CO at high pressure. For the purification of nickel The Mond process relies on the formation of nickel tetracarbonyl Ni (CO)4 in this method, followed through its thermal decomposition to deposit metallic nickel. For previous elements in the series reductive carbonylation is needed, with a compound (usually a halide) reduced in the existence of CO at high pressure. Polynuclear carbonyls are created naturally for some elements (Mn, Co); in other examples, like Fe in which the mononuclear carbonyl Fe(CO)5 is stable, polynuclear compounds can be prepared from it through photolysis or controlled
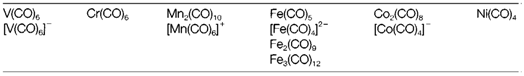
pyrolysis. Binary carbonyls are volatile compounds, frequently very toxic, and thermodynamically not stable in the existence of oxygen but frequently with significant kinetic stability, particularly for metals later in the series.
In mononuclear carbonyls CO is always attached to the metal by carbon providing a linear M-C-O arrangement. Polynuclear carbonyls contain comparatively short distances among metal atoms indicative of metal-metal bonds. CO can then bond within either a terminal or a bridging mode, the previous bonded to one metal like in Mn2(CO)10 (1) and the latter attached to more than one metal like in Co2(CO)8 (2). In larger clusters created through some elements, triply bridging CO is also probable. Terminal and bridging CO might be distinguished through IR spectroscopy, like bridging groups depict a characteristically lower stretching frequency.

Several compounds are known containing CO in conjunction along with other ligands, that may include π acceptors like phosphines, and/or a bonding ligands. For instance, there is a series of compounds Mn(CO)5X, in which X=H, halogen or an alkyl group.