16-electron complexes
A square-planar complex of a d8 ion, like [Ni(CN)4]2-, have a valence electron count of 16 rather than 18. Identical 16- electron complexes are created through other elements in groups 9, 10 and 11, for instance Vaska's compound Ir(CO) (PPh3)2Cl (3). Some 16-electron complexes (particularly in the 3d series) can readily add other ligand to create a five coordinate 18-electron complex like [Ni(CN)5]3-. Other significant reaction is termed as oxidative addition, in which a molecule X-Y adds by cleavage of the bond to create an 18-electron complex which can be considered as d6 octahedral:
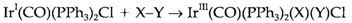
X-Y could be a simple molecule like H2 or HCl, or an organic compound. Vaska's compound also reacts along with O2 to create Ir(CO)(PPh3)2(O2)Cl (4). In this example, O2 remains intact on coordination, even though the bond lengthens, suggesting that 4 can be considered as a complex along with a bidentate peroxo ligand.

The opposite of oxidative addition is reductive elimination. Such type of reversible processes is significant in several catalytic cycles involving transition metal compounds.