Paramagnetism
In diamagnetism substances are repelled through a magnetic field: this property is related with all closed electron shells. Paramagnetic substances are involved into a magnetic field, the force being associated to the magnetic susceptibility. Paramagnetism generally occurs from the spin of unpaired electrons. The Curie law for the susceptibility per mole (χm) is
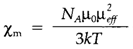
In which NA is Avogadro's number, μ0 of free space the magnetic permeability, μeff the efficient magnetic moment of the paramagnetic species, k is Boltzmann's remain unchanged and T the temperature in kelvin. The opposite temperature dependence arises since thermal agitation works against the alignment of moments in an applied field. For several d-block compounds the spin-only formula is a comparatively good approximation to the effective magnetic moment:
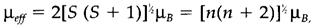
In which S is the spin quantum number, equivalent to half the number of unpaired electrons n, and μB the Bohr magneton, equivalent to about 9.274×10-24 J T-1. The simplest application of magnetic measurements is thus to establish the number of unpaired electrons, and thus to differentiate between high- and low-spin states. For instance, most Co3+ complexes contain μeff=0 as supposed for low-spin d6; though, [CoF6]3- has μeff approximately 5μB, subsequent to four unpaired electrons and a high-spin state.
Magnetic measurements are occasionally used to provide information about metal-metal bonding. For instance, dimeric Cr2+ complexes like [Cr2(CH3CO2)4] contain μeff=0, suggesting that all four d electrons of Cr2+ are paired to create a quadruple bond. Though, there are several other issues that can complicate magnetic properties. The oxygen-bridged complex [(RuCl5)2O]4- also has μeff=0. There is no metal-metal bond and the electrons are paired like a consequence of the Ru-O bonding in this case.