Electronic transitions
An electron is excited from an occupied to an empty molecular orbital (MO) in an electronic transition. The energy of such type of transitions generally corresponds to photons in the near IR, visible or UV area of the electromagnetic spectrum.
Electronic absorption bands provide rise to the colors of compounds, involving ones without transition metals.
In d-block complexes several types of MO can be included. In d-d transitions both the lower and upper MOs are those that are based on the d atomic orbitals, split through interaction along with the ligands. Charge transfer transitions include ligand-based MOs too, and might be divided into ligand-to-metal charge transfer (LMCT, the most common type) or metal-to-ligand charge transfer (MLCT). There might also be transitions among two ligand MOs (example n to π* in unsaturated ligands). ligand-based transitions and charge transfer frequently come out at higher energy than d-d transitions, and are usually also more intense. Figure 1 depicts the absorption spectrum of [Ti(H2O)6]3+. The d-d transition peaks at around 20 000 cm-1 (500 nm) subsequent to green light, providing a violet color to the complex (transmitting red and violet light). The strong absorption increasing beyond 25 000 cm-1 is because of LMCT.
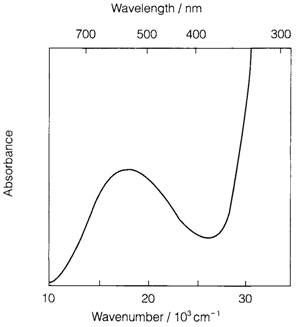
Fig. 1. Absorption spectrum of [Ti(H2O)6]3+..
d-d spectra d-d transitions are weak due to atomic selection rules, that make transitions among d orbitals forbidden.
They stay forbidden in complexes with a center of symmetry (example octahedral or square planar), and come out only due to vibrational motions which break this symmetry. In complexes with no a center of symmetry (example tetrahedral) the transitions are stronger but are weak still as compared with charge transfer. In addition there are also spin selection rules, the strongest transitions being spin-allowed ones in which there is no modification in the number of unpaired electrons.
Excitation of an electron from t2g to eg provides a single absorption band at an energy equivalent to the ligand field splitting Δo , in a d1 octahedral complex like [Ti(H2O)6]3+. The theory is very complex for ions with several d electrons since the energy of a state is now determined through the repulsion among electrons with the occupancy of t2g and eg orbitals. The supposed number of spin-allowed d-d transitions in high-spin octahedral or tetrahedral complexes is displayed below. Not all transitions might be noticeable in all cases, since bands may overlap or some may be obscured through charge transfer:
one for d1, d4, d6 and d9;
three for d2, d3, d7 and d8;
none for d0, d5 and d10.
The nonexistence of spin-allowed transitions for high-spin d5 can be recognized from the fact that in ground state all d orbitals are singly taken through electrons that contain parallel spin. This is the single possible state along with five unpaired electrons, and some d-d transition might include a change of spin, d-d transitions with in high-spin Mn2+ and Fe3+
complexes are certainly extremely weak compared with other ions, that have spin-allowed transitions.
A mathematical analysis of the transition energies within the dn ions permits Δo to be determined with electron repulsion parameters. Electron repulsion among d electrons in complexes is found to be less than within the free gasphase dn ions. This type of reduction is called the nephelauxetic effect (mean 'cloud expanding') and takes place since 'd orbitals' in complexes are actually MOs with a number of ligand with metal contribution, thus that electrons are on average more separately than in the pure d orbitals of the uncombined ions. Larger nephelauxetic reductions are discovered in complexes with 'soft' ligands like I- than with 'hard' ones such F-, that are reflecting the greater degree of covalent bonding with in the former case.