Charge transfer spectra
Charge transfer is similar to an internal redox reaction and the absorption energies could be related with trends in redox properties. An electron is transferred to the metal in LMCT, so that is reduced in the excited state. The more positive the redox potential apprehensive, the easier such type of reduction will be, and thus the lower the LMCT energy. LMCT transitions in the noticeable region of the spectrum provide intense color, as is found with permanganate MnO-4 a d0 complex, so this therefore has no d-d transitions. The energy trends in a number of d0 species are:
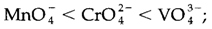
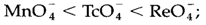
That follows the trends in the direction of less strongly oxidizing compounds,
(i) In the direction of the left in the 3d series and
(ii) down each transition metal group. The above orders of LMCT energy are reflected within the changing colors of the ions (example MnO-4deep purple, CrO2-4 deep yellow, VO3-4pale yellow, like the transition moves increasingly to higher energy out of the noticeable spectrum into the UV).
LMCT energies also follow usual trends as the ligand is changed, for instance, O>S, and F>Cl>Br, as the heavier ions within each group are more simply oxidized. With dissimilar metal ions, there is a usual decrease in energy in the direction of the right in every series. For ions in lower oxidation states, LMCT frequently takes place in the UV rather than the visible part of the spectrum.
MLCT is less general, as it needs the presence of empty ligand orbitals of appropriate energy. Several of these ligands are π acceptors. With changing metal ions and oxidation states, MLCT bands frequently follow the opposite of the trends found with LMCT.