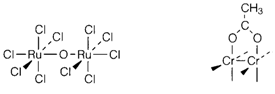Coordination number and geometry
Transition metal complexes are cationic, neutral or anionic species where a transition metal is coordinated through ligands. A classical or Werner complex is one created through a metal in a positive oxidation state along with donor ligands like H2O, NH3 or halide ions.
The coordination numbers (CN) discovered in complexes range to nine (e.g. [ReH9]2- from two (e.g. [Ag(NH3)2]+);). The most common geometries for 3d ions are octahedral (CN=6, example [M(H2O)6]2+) and tetrahedral (CN=4, example [MCl4]2-). Like in solid compounds, higher coordination numbers are frequently found with the larger 4d and 5d ions. Other Coordination geometries might be dictated through bonding arrangements relies on the d electron number.
The comparative preference for octahedral or tetrahedral coordination is fairly steric, but ligand field influences can also play a vital role. Ions along with the d3 and low-spin d6 configurations (examples Cr3+ and Co3+, respectively) comprise large octahedral ligand field stabilization energy and are particularly resistant to creating tetrahedral complexes. Square-planar complexes would never be supposed in preference to tetrahedra on steric grounds alone. Though, they are generally found with 4d8 and 5d8 ions like Pd2+ and Pt2+ in which the pattern of ligand field splitting is favorable if its magnitude is sufficiently large for spin-pairing to happen. The subsequent 3d8 ion Ni2+ gives square-planar complexes just with strong-field ligands like CN-; or else octahedral or occasionally tetrahedral coordination is found. Along with the d9 or high-spin d4 configuration a deformed octahedral geometry is frequently found with just four ligands strongly attached. This is general with Cu2+, like in [Cu(NH3)4]2+, in which two weakly bound water molecules are also exist.
Low coordination numbers are frequently found with post-transition metal ions that are having the d10 configuration. This is also right for the d10 ions Cu+, Ag+ and Au+, that form several linear complexes with CN=2 (example [AuCl2]-, isoelectronic to HgCl2).
Polynuclear complexes have more than one metal atom. Occasionally these might be held through bridging ligands, like in [(RuCl5)2O]4- (1). In another cases metal-metal bonds might be exist, like in [Re2Cl8]2-. Metal-metal bonding is common within the 4d and 5d series than with 3d elements, even though binuclear compounds of CrII are well-known; for instance, [Cr2(CH3CO2)4] (2), that has bridging acetate groups (only one displayed explicitly) and a quadruple Cr-Cr bond created through all remaining valence electrons of the 3d4 ions.