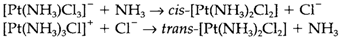Square-planar complexes
Kinetically inert square-planar complexes are created through d8 low-spin ions, particularly Pt2+. Ligand substitution is associative and related with the easiness of forming a five-coordinate transition state (or intermediate). Substitution is very much faster with Ni2+ in which five-coordinate complexes like [Ni(CN)5]3- are very much stable than for Pt. For a particular metal, the rate of substitution is controlled through:
- The nature of the leaving and incoming ligands; more polarizable groups are usually faster in both bond-making and breaking processes;
- The trans effect that is the ability of a number of ligands to facilitate the substitution of the ligand trans to them in the complex. A few ligands in order of increasing effectiveness are:
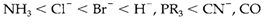
The trans effect is a kinetic occurrence and is affected through different factors that operate either in the ground state or in the five-coordinate transition state. A number of ligands weaken the bond trans to them with in the original complex. This ground- state phenomenon is called the trans affect and depends mainly on the σ bonding polarizability and capability of the ligand. Some ligands like CN- do not depict much trans affect but yet have a large kinetic trans influence, since their π-acceptor properties help out in the stabilization of the transition state.
The trans effect is helpful in synthesis. For instance, different isomers are created in the reactions below through the greater trans directing capability of Cl- compared with