Octahedral Complexes
Several M2+ ions of the 3d series go through ligand exchange at a rate comparable with that for nontransition metal ions of identical size. V2+ (d3) and Ni2+ (d8) are rather slower, these being the electron configurations which provide maximum octahedral LFSE for high-spin ions. Volumes and Entropies of activation imply a change from predominantly Ia techniques early in the series (example V2+) to Id in the direction of the end (e.g. Ni2+). Both decreasing size and increasing d orbital occupancy might contribute to this tendency. Incoming ligands in the Ia mechanism might be approach an octahedral complex along directions in which the t2g orbitals generally point. Filling these orbitals will tend to reduce the approach of ligands and favor the dissociative pathway.
For the kinetically inert low-spin CoIII complexes the technique of exchange is specifically dissociative even though kinetic studies can provide results which are super-ficially misleading. For instance, the base hydrolysis reaction
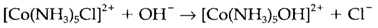
Have a rate proportional to the concentrations of both complex and OH-. This is not suggestive of an associative technique, but of a conjugate base technique in which the first reversible step is deprotonation of the complex:
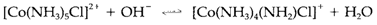
Deprotonation trans to the leaving group is particularly efficient at promoting the dissociation step. The conjugate base technique cannot operate if a tertiary amine along with no ionizable proton is located trans to the leaving group; as supposed the rate of substitution is then slower and does not rely on [OH-].