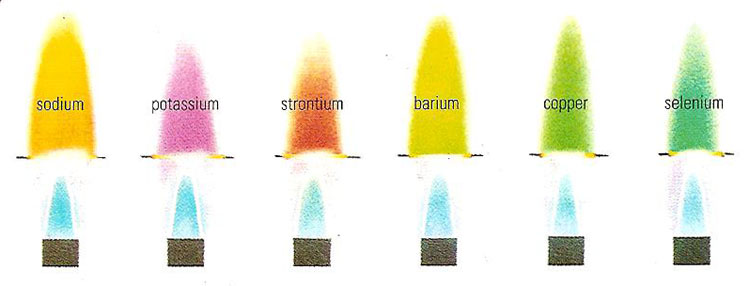
Flame Test
A flame test is carried out to determine the identity of an unknown metal ion based on the characteristics colour the salt turns the flame of a Bunsen burner.
A platinum or nichrome wire is taken and then cleaned by dipping it in concentrated hydrochloric acid followed by rinsing it with the distilled water. This is then introduced in the Bunsen flame to test the contamination. If the wire is not contaminated, the color of flame will not change.
If the changes occur in flame colour, dip wire in the concentrated hydrochloric acid again and then return it to the Bunsen flame.
Repeat this procedure until the wire shows no change of colour in the flame
The loop is then dipped in either powder or solution of the salt and then put in a Bunsen flame. The resulting flame color provides the identity of the metallic ion (cation) in the salt.