Enzyme catalysis and Michaelis constant
A catalyst is a substance which has the ability to accelerate the chemical reaction without being altered or consumed in the process. Enzymes are biological catalysts made from protein molecules mostly. Michaelis-Menten enzyme kinetics is a model for rate equations that has a closed-form solution for concentrations of the products and reactants in an enzymatic reaction. Enzymatic catalysis can enhance the reaction rate by a factor. The study of reaction rates in chemical reactions that are catalyzed by enzymes is called as enzyme kinetics. Enzymes act by decreasing the activation energy. They don't change the equilibrium of the reactions. Enzyme binds with the substrate to form the unstable complex which breaks up into the products liberating enzymes. The small region on the enzyme where the substrate binds and catalysis takes place is known as active site. The Michaelis-Menten mechanism for the enzyme kinetics can be given by:
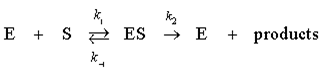
here E is the enzyme, S is the substrate, and ES is an enzyme-substrate complex.
The Michaelis-Menten equation describes the relationship in between the rate of substrate conversion by the enzyme to the concentration of the substrate.
V= Vmax [ S] / Km + [S]
here Vmax is the maximum enzyme velocity. It is the velocity of the enzyme extrapolated to the high concentrations of the substrate and its value is almost always higher than any velocity measured in your experiment. It is the maximum rate of conversion. Km is the Michaelis-Menten constant. It is the concentration of substrate required to achieve a half-maximum enzyme velocity. It approximates the affinity of enzyme for substrate. When the reaction rate becomes independent of the substrate concentration the rate is said to be zero order. The remarkable specificity of enzymes comes from the ability of the enzymes to bring the substrates into a favorable orientation in the enzyme-substrate (ES) complexes so that the substrates are bound to specific regions of the enzymes. Each enzyme has a some specific active site. The active site of an enzyme binds the substrate and contains the residues which directly participate in the making and breaking of bonds.