Coordinate covalent bond / Dative bond:
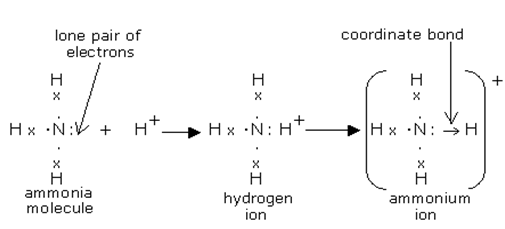
In the covalent bonding we have seen that the 2 electrons taking part in the bonding are supplied by 2 different atoms. In case of coordinate covalent bond both the electrons taking part in the covalent bond are supplied by the same atom.
For example, when ammonia is dissolved in water nitrogen in the molecule donates one of its lone pair (valence electrons that are not taking in any chemical bond) of electrons to one hydrogen ion (proton) present in water. The hydrogen then uses the donated electron to bond covalently with the nitrogen molecule. In this bond both the electrons are supplied by the same atom, nitrogen.
Other examples of coordinate covalent compounds include carbon monoxide, ammonia-borontrifluoride complex etc.