Linked polyhedra
A different way of analyzing binary structures is to concentrate on the coordination polyhedra of one type of atom and on the way these are related together. This approach is usually helpful in structures with covalent bonding and/or ones that are more open than those derived from close packing. If the two tetrahedral AB4 units divide one B atom in common (1) we talk of corner sharing. A corner-shared pair has a stoichiometry A2B7 and is found in (molecular) Cl2O7 and sometimes in silicates. Tetrahedra every sharing corners with two others generate a ring or chain (2) of stoichiometry AB3, as found with SO3 and generally in silicates. These structures are frequently represented by drawing the tetrahedra without depicting the atoms explicitly. Chains and rings with two corners shared are displayed in this way (Fig. 3a and b). Sharing three corners forms a tetrahedral cluster of stoichiometry A2O5 or a layer; such types of layers take place in silicates and the clusters as P4O10 molecules. Tetrahedra sharing all four corners with others generate a 3D framework of stoichiometry AB2, found in the several (glassy and crystalline) structures of SiO2.
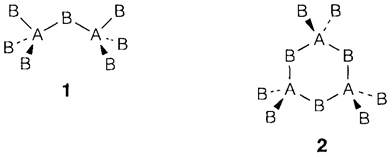
Commonly, tetrahedra with two B atoms are said to be edge sharing: instances of isolated edge-sharing pairs are B2H6 and Al2Cl6. A chain of tetrahedra each sharing two edges with others has a stoichiometry AB2 and is found as the chain structures of BeH2 and SiS2, displayed in Fig. 3c. Face sharing is also feasible but is nearly never found with tetrahedra like the A atoms would be very close together.
Identical ideas can be used with octahedra. Corner-sharing octahedra's chains are found in WOBr4 and of edgesharing octahedra in NbI4. The 3D ReO3 structure results, if octahedra share all six corners.
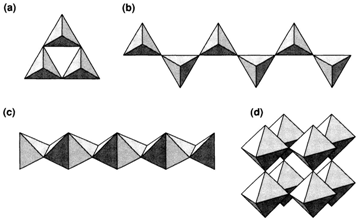
Fig. Structures derived from linking of polyhedra (see text).