Close packing
Several binary structures can be derived from close-packed arrays of atoms of one kind. Figure 2 depicts that between adjacent close-packed layers are tetrahedral and octahedral holes (labeled T and O) like that atoms of another kind occupying these sites would be tetrahedrally or octahedrally coordinated. We can imagine the larger ions (generally the anions) forming the close-packed array for ionic compounds and cations taking place some of the holes. In either cubic close-packed (ccp or fcc) or hexagonal (hcp) arrays of B there is one octahedral and two tetrahedral holes per B atom. Table 1 depicts some binary structures classified in this way. So filling all the octahedral holes in an fcc array generates the rocksalt structure (where the original B atoms are also octahedrally coordinated); doing similar in an hcp array gives the NiAs structure. Filling all tetrahedral holes in an fcc anion array gives the antifluorite structure, more generally found with cations and anions reversed as in fluorite (CaF2) itself. A identical arrangement is never found in an hcp array, as the tetrahedral holes take place in pairs that are very close together.
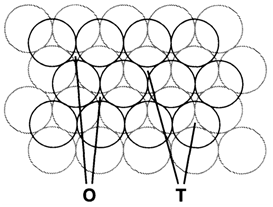
Fig. 2. Octahedral (O) and tetrahedral (T) holes between adjacent close-packed layers.
Table 1. Some binary structures based on close-packed arrays of anions
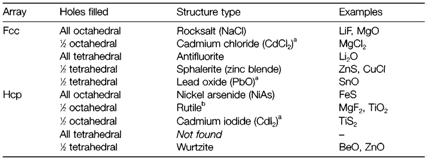
a Layer structures.
b Filling the holes changes the symmetry; rutile unit cell is not hexagonal.
While only a fraction of the holes of a particular type are occupied there are various possibilities. The most symmetrical method of filling half the tetrahedral holes gives the zinc blende structure with ccp and the very identical 4:4 wurtzite (ZnO) structure with hcp. Both the CdI2 and rutile structures can be derived by filling half the octahedral holes in hcp. The former provides a more regular coordination of the anions (see above) even though the resulting structure is no longer hexagonal. The CdI2 structure occurs from alternately occupying every octahedral hole among two adjacent closepacked planes and leaving the next layer of holes empty. It is an instance of a layer structure based on BAB 'sandwiches' that are stacked with only B-B contacts among them. The CdCl2 structure is based in a identical way on ccp (rather than hcp) anions and several other layer structures with formulae like AB3 can be formed by only partial filling of the holes among two layers.