Q. What do you mean by Electrolysis?
In electro refining, the impure metal is taken as the anode and a strip of pure metal coated with a thin layer of graphite is made .the cathode in an electrolytic cell. The electrolytic is an aqueous solution of a salt of the metal. On electrolysis, the impure metal from the anode goes into solution and metal ions are reduced and get deposited on the cathode. Only weakly electropositive metals like copper, tin and lead which are readily oxidised at the anode and reduced at cathode can be purified in this manner. A general reaction can be written as follows:
M( impure) -------------> Mn+ (aq) + e at anode
Mn+ (aq) + ne ----------> (pure) at cathode
Other impurities in the metal settle downas anode mud or remain dissolved in the, solution.
In the case of electrolytic refining of copper, an impure copper rod is made the anode, pure
copper strip the cathode and copper sulphate solution the electrolyte (Fig. 15.7). The following electrode reactions take place:
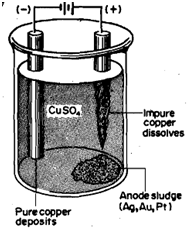
Cu(s) ------------> Cu2+ (aq) + 2e e at anode
Cu2+ (aq) + 2e -------------> Cu (s) at cathode
Thus, 99.95% pure copper is obtained in this process. The more reactive metals such as iron which are present in the crude copper are also oxidised at anode and pass into solution. The voltage is so adjusted that they are not reduced at cathode and thus remain in solution. The less reactive metals such as silver, gold and platinum if present, are not oxidised. As the copper anode dissolves, they fall to the bottom of the cell from where they are recovered as a valuable anode mud.