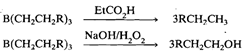Q. Show the Properties of diborane?
Diborane is a colourless gas (b. p., 183K). It is rapidly decomposed by water with the formation of H3B03 and H2:
B2H, + 6H20 ---------------------------------------->2H3,BO3, + 6H2
Mixtures of diborane with air or oxygen inflame spontaneously producing large amount of heat. Diborance has a higher heat of combustion per unit weight of fuel than most other fuels. Therefore it is used as a rocket fuel.
B2H6+ 3O2---------------------------------> B2O3+ 3H2O, DH= -2165 mol-1
Pyrolysis of B2H, in sealed vessels at temperatures above 375 K is an exceedingly complex process producing a mixture of various boranes, e.g., B4H1o, B5H9, B5H12 B6H1o, B4H12 and B1oH14+ By careful control of temperature, pressure and reaction time, the yield of various intermediate boranes can be optimised. For example, by storing B2H6 under pressure for 10 days B4HI10 is produced in 15% yield according to the following equation:
2B2H6------------------> B4H10 + H2
Diborane undergoes a facile addition reaction with alkenes and alkynes in ether solvents at room temperature to form organoboranes:
This reaction known as hydroboration reaction was discovered by Brown and Subba Rao in 1956. It is region specific, boron atom showing preferential attachment to the least substituted carbon atom. You may compare this addition with polar additions to the double bond, e.g., addition of HX, which obey Markownikoff's rule. Reaction of the resulting organoborane with an anhydrous carboxylic acid yields the alkane corresponding to the initial alkene whereas oxidative hydrolysis with alkaline H202 yields the corresponding primary alcohol: