Refer to diagram A, which describes the system formed by water-ethanol mixtures at 1 atm pressure, but is not drawn to scale. Industrial ethanol production results in the lowest-boiling water-ethanol mixture. This lowest-boiling mixture is normally about 95.7 % ethanol. Its boiling point is about 78.2 ºC, whereas pure ethanol boils at 78.5 ºC.
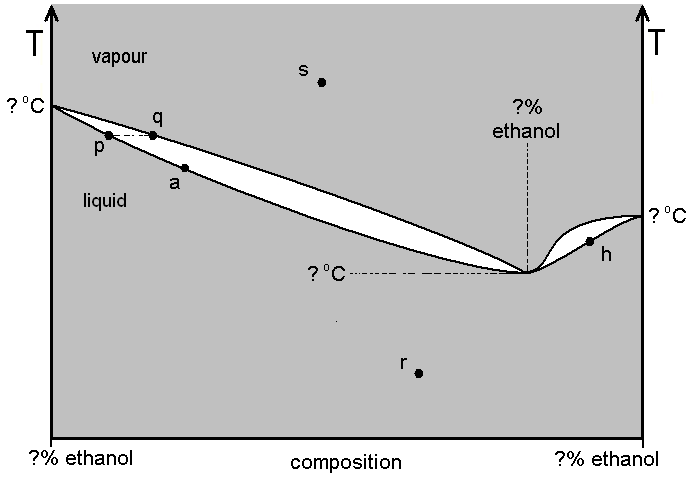
Diagram A
(a) Carefully detach the copy of the diagram from the back of this paper and attach it to your answer book. Enter on the diagram any value marked by a question mark "?" (there are six of them).
(b) Name the mixture which consists about 95.7 % ethanol, 4.3 % water. Is the ethanol-water system one displaying 'negative deviation' or 'positive deviation'?
(c) Normally, in the industrial process, a "wash" (a dilute solution of ethanol, represented by point a on diagram A), is distilled using an efficient fractionating column. Assuming a fractionating column operates as a system of repeated distillations, describe how a product of 95.7 % ethanol composition can be achieved. Enter letter symbols on your diagram to refer to, starting from point a. Would the 95.7 % ethanol solution be recovered at the top of the column, or from the flask?
(d) It is often wrongly assumed that 95.7 % is the greatest concentration of ethanol attainable industrially, starting from a "wash". However, in practice, the 95.7 % solution can further be dehydrated using vigorous dehydrating agents (such as CaO) to give a solution approximately 97% ethanol (marked by h on the diagram). Show how, if a sufficient amount of 97% solution was to be poured into a flask topped by an efficient fractionating column, further fractional distillation might give amounts of highly pure ethanol. At the end of this process, would the ethanol be in the flask, or recovered at the top of the column? What would be the other product of this process? Use and amend the supplied diagram as necessary to help explain your answer.
(e) Using the 'Phase Rule', calculate the absolute degrees of freedom for the systems denoted by the four points p, q, r and s.