From the EPA the current generation of methane from landfills in the USA is 12.7 MM tons per year (2009).
a. Using the Global Warming Potential of the methane at 20 years, 100 years and 500 years, calculate the equivalent CO2 contribution of the methane generated by the US landfills.
The GWP of methane at different horizons is provided by the table in Introduction.
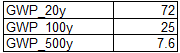
Knowing the emissions of methane by landfills we can therefore calculate the equivalent emissions of CO2 at the different horizons:

b. When methane from a landfill is flared the methane is burnt following the equation:
CH4 + 2O2 -> CO2 + 2 H2O
How much kg of CO2 is emitted by the combustion of a kg of methane?
Using the atomic mass of elements and the stoichiometry of the reaction, we show that every kg of CH4that is burnt generates 2.75 kg of CO2.
c. Compare the equivalent CO2 contribution of the methane when released to the atmosphere at 20 years, 100 years and 500 years with its contribution when burned, in kg CO2,equ per kg of CH4.
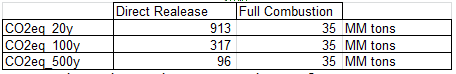
d. Is it better to burn the methane or to release it?
Results of question c. clearly show that it is much less harmful to burn the methane than to directly release it in the atmosphere.
e. From EPA, about 7.83 MM tons of methane is burned per year in the US landfills. The rest is release directly to the atmosphere. Calculate the annual contribution of the methane generated from landfills in the US in equivalent CO2 at 20 years, 100 years, 500 years.
Using the calculation methods of a., b. and c. we can establish:
f. In percent, what is the contribution of the released methane compared to the its total contribution in equivalent CO2 at 20 years, 100 years, 500 years?
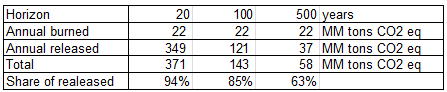
The last row of the table presented in e. gives the share in CO2 eq of the direct release of methane currently performed in the USA. We observe that, even if more than 50% of the methane generated in landfills is burnt today, the contribution to global warming of directly released methane is dominant for all time horizons.