Q. Describe about tetrahalides?
S, Se, Te and Po form tetrahalides with all the halogens. However, tetrafluoride of polonium, tetrabromide of sulphur and tetraiodides of sulphur and selenium are not formed. The tetrahalides are formed mainly by the reaction between the corresponding elements, e.g.,
Te + 2Cl2 ------------> TeCl4
These are covalent compounds which hydrolyse to give the corresponding dioxides.
SeBr4 + 2H2O--------------> SeO2+ 4HBr
SF4+ 2H2O-------------------> SO2 + 4HF
As stated' above, the tetrahalides dissociate in solution or in the vapour state to the corresponding dihalides.
SeCl4----------------------------> SeCl2 + Cl2
The tetrahalides also form complex anions of, the type EX2-6 in the presence of excess of the corresponding halogen acid.
SeCl4 + 2HCl -----------------------------------> H2SeCl6
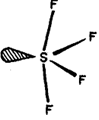
There has been much controversy concerning the structure of tetrahalides. The presently accepted structure of SF4, and SeF4, is see-saw with one equatorial position occupied by an electron pair as shown in Fig.