Reference no: EM132091333
Complete the following exercises.
1. Which of the following are physical properties? (Select all that apply)
A. Color
B. Hardness
C. Density
D. Flammability
E. Conductivity
F. Reactivity with other materials
2. Which of the following describe a physical change? (Select all that apply)
A. Photosynthesis
B. Evaporation of alcohol when a table is wiped with an alcohol swab
C. Burning of firewood
D. Rusting of iron
E. Breaking a glass
F. Melting ice
3. Which of the following are chemical properties? (Select all that apply)
A. Texture
B. Toxicity
C. Conductivity
D. Density
E. Flammability
F. Reactivity with other materials
4. Which of the following describe a chemical change? (Select all that apply)
A. Melting of ice
B. Evaporation of alcohol when a table is wiped with an alcohol swab
C. Burning of firewood
D. Rusting of iron
E. Breaking a glass
F. Cooking an egg
5. Match the following substances with the best classification:
1. Magnesium
2. Salt water
3. Blood
4. Carbon dioxide
5. Soil sample
A. Compound
B. Element
C. Solution
D. Heterogeneous mixture
E. Suspension
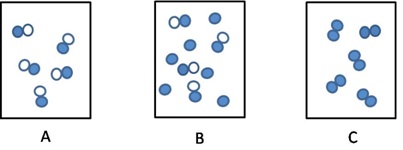
6. Which of the following illustrations best illustrate an element, a compound, and a mixture. Provide an explanation for your choices.
7. Describe a method to separate the substances in a solution.
8. Distinguish between a saturated and an unsaturated solution.
9. Match the following:
1. Sodium chloride, NaCl
2. Oxygen, O2
3. Methane, CH4
4. Lithium
5. Air
A. Element
B. Solution
C. Diatomic gas
D. Ionic compound
E. Covalent compound
10. Electron dot diagrams are a way to illustrate the number of valence electrons for an element. The symbol for the element is surrounded by a dot for each valence electron. Match the following electron dot diagrams with an element:

1. Electron dot diagram #1
2. Electron dot diagram #2
3. Electron dot diagram #3
4. Electron dot diagram #4
A. Sodium, Na
B. Aluminum, Al
C. Flourine, F
D. Nitrogen, N
11. For the following questions consider a compound of calcium and chlorine.
a. What type of bond do calcium and chlorine form?
b. Describe how this bond forms.
c. What is the chemical formula for the compound? Explain.
12. For the following questions consider a water molecule, H2O.
a. What type of bond is formed between the oxygen and the hydrogen atoms?
b. Describe how these bonds form.
c. Explain why there are two hydrogen atoms for each oxygen atom.
13. For the following questions consider a piece of copper wire.
a. What type of bond is formed between the copper atoms?
b. Describe how these bonds form.
c. Explainwhy copper is a good electrical conductor.
14. Explain why food stays fresh longer when kept in the refrigerator.
15. In the stratospheric level of the atmosphere there is a high level of ozone, O3. This ozone absorbs much of the ultraviolet light from the sun and thus protects life in the surface of the Earth from harmful high level of ultraviolet radiation. Explain why one chlorofluorocarbon (CFC) molecule in the ozone layer is capable of breaking down hundreds and even thousands of ozone molecules?
16. Balance the following chemical reaction by placing the proper coefficients in front of each reactant and in front of the product.

a. What is the coefficient on line (a)?
b. What is the coefficient on line (b)?
c. What is the coefficient on line (c)?
17. Acetylene gas (C2H2) burns in the oxyacetylene torch for welding. Balance the chemical reaction by placing the proper coefficients in front of each reactant and each product.
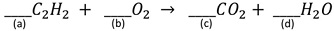
a. What is the coefficient on line (a)?
b. What is the coefficient on line (b)?
c. What is the coefficient on line (c)?
d. What is the coefficient on line (c)?
The remaining questions are multiple-choice questions:
18. What is a substance made up of only one type of atom called?
A. An element
B. A mixture
C. A solution
D. A compound
E. A nucleus
19. Which of the following is a solution?
A. Table salt
B. Nitrogen
C. A vinegar and oil salad dressing
D. Copper wire
E. A 14 karat gold ring
20. Which of the following is a heterogeneous mixture?
A. Table salt
B. Air
C. A vinegar and oil salad dressing
D. Steel
E. A 14 karat gold ring
21. On a container of chicken broth you notice the instructions to "shake well before serving." Which of the following best describes the chicken broth?
A. A solution
B. An element
C. A compound
D. A suspension
E. A pure substance
22. The formation of an ionic bond involves:
A. A transfer of electrons
B. A transfer of protons
C. A transfer of neutrons
D. A sharing of electrons
E. A sharing of protons
23. Which of the following is the chemical formula for the ionic compound magnesium nitride?
A. MgN
B. Mg2N
C. Mg2N3
D. Mg3N2
E. MgN2
24. Which of the following is correct about a molecule of carbon trichloride?
A. It has one carbon atom for each chloride atom.
B. It has three chloride atoms for each carbon atom.
C. It has three carbon atoms for each chloride atom.
D. It is a mixture of equal parts carbon and trichloride.
E. It is a solution of carbon in chlorine.
25. What is the term for the substances that undergo a change in a chemical reaction?
A. Solutions
B. Products
C. Solvents
D. Catalysts
E. Reactants
26. When methane (CH4) burns it combines with oxygen and forms carbon dioxide and water. Which of the following is the balanced chemical reaction for the burning of methane?
A. CH4 + O2 → CO2 + H2O
B. 3 CH4 + 6 O2 → 3 CO2 + 6 H2O
C. 2 CH4 + 3 O2 → CO2 + 4 H2O
D. CH4 + 3 O2 → 2 CO2 + 2 H2O
E. CH4 + 2 O2 → CO2 + 2 H2O