Boyle's law:
|
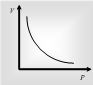
At a fixed temperature, the volume of the given mass of gas is inversely proportional to its pressure.
P ∝ 1/V or PV = constant or P1V1 = P2 V2. This law is perfectly ruled at high temperature and low pressure of a gas.
|
|
Charle's law
|
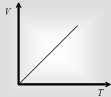
At fixed pressure the volume of a given mass of gas is directly proportional to its absolute temperature.
V ∝ T or V/T = Constant => V1/T1=V2/T2 => V1T2 = V2T1 T1Ρ1= T2 Ρ2
At constant pressure the volume of a given man of gas increases by 1/273 of its volume of 0°C for 1°C gain in its temperature.
Vt = Vo[1+ (1/273)t]
|
|
Gay Lussac's law:
|
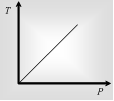
Gay Lussac's law on kelvin scale defines that volume having constant, the pressure of the required mass of a gas is directly Proportional to its kelvin temperature.
P ∝ T . P1/T1=P2T2
It said that the volume remaining fixed, the pressure of a given man of a gas decrease or increases by 1/273.15 of its pressure at 0°C for each 1°C rise or fall in temperature Pt = Po{1+(1*t)/273.15}
|
|
Dalton's law of partial pressure:
The net pressure exerted by the mixture of in passive gases in a vessel is same to the sum of partial pressure due to individual gases P = P1 + P2 +P3 .......
Avogadro's law:
It defines that same volumes of all gases under identical conditions of pressure and temperature, contain the similar number of molecules. If P1 = P2 ,V1 = V2, T1 = T2 then n1 = n2
Graham's law of diffusion:
It defines that the rates of diffusion of two gases are inversely proportional to the square roots of their densities r ∝1/√Ρ; Where r = Rate of diffusion, Ρ = density of gas.
Ideal gas equation:
It is a relation which relates to the pressure, temperature and volume of the given state of an ideal gas.
|
PV = RT
|
for 1 mole of gas
|
PV = nRT
|
for n mole of gas
|
|
PV = rT
|
for 1 gram of gas
|
PV = nrT
|
for n gram of gas
|
|
PV = KT
|
for 1 molecule of gas
|
PV = nKT
|
for n molecule of gas
|
R = universal gas constant; r = specific gas constant; k = Boltzmann's constant ; P = pressure
V = volume; T = temperature
Email based Physics assignment help - homework help at Expertsmind
Are you searching physics expert for help with Various laws for gases questions? Various laws for gases topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Physics assignment help and physics homework help. Live tutors are available for 24x7 hours helping students in their Various laws for gases related problems. We provide step by step Various laws for gases question's answers with 100% plagiarism free content. We prepare quality content and notes for Various laws for gases topic under physics theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving physics queries in excels and word format.
- Best tutoring assistance 24x7 hours