Van't Hoff's factor (i) : In the year 1886, Van't Hoff introduced the factor 'i' called Van't Hoff's factor, to express the extent of the association or dissociation of the solutes in solution. It is the ratio of the normal and observed molecular masses of solute, that is
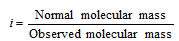
In the case of association, observed molecular mass being more than normal, the factor i has a value less than 1. But in case of the dissociation, the Van't Hoff's factor is more than 1 because observed molecular mass has a lesser value than the normal molecular mass. In case there is no dissociation value of 'i' becomes equal to 1.
As colligative properties are inversely proportional to the molecular masses, the Van't Hoff's factor can also be written as follows
,

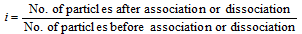
The introduction of the Van't Hoff factor modifies the equations for the colligative properties as written below,
Relative lowering of vapour pressure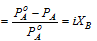
Elevation of boiling point, Tb = ikbm
Depression in freezing point, Tf = ikfm
Osmotic pressure, 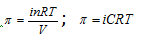
From value of 'i', it is possible to calculate the degree of dissociation or the degree of association of substance.
The degree of dissociation (a): It can be defined as the fraction of the total molecules which dissociate into the simpler molecules or ions.
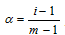 ; m= number of particles in the solution
; m= number of particles in the solution
The degree of association (a): It can be defined as the fraction of the total number of molecules which associate or combine together resulting in formation of the bigger molecules.
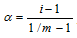 ; m = number of particles in the solution.
; m = number of particles in the solution.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Van’t Hoff’s factor questions? Van’t Hoff’s factor topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Van’t Hoff’s factor related problems. We provide step by step Van’t Hoff’s factor question's answers with 100% plagiarism free content. We prepare quality content and notes for Van’t Hoff’s factor topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours