Theories of reaction rate
(1) Collision theory
(i) The basic requirement for a reaction to occur is that the reacting species must collide with one another. This is support the collision theory for reactions.
(ii) The number of collisions which takes place per second per unit volume of the reaction mixture is called ascollision frequency (Z). The value of the collision frequency is quite high of the order of 1025 to 1028 in case of the binary collisions.
(iii) Every collision does not bring the chemical change. The collisions which in reality produce the product areeffective collisions. The collisions which are effective bring chemical change, are less in the comparison to the total number of collisions. The collisions which do not result in product are ineffective elastic collisions, that is molecules simply collide and disperse in different directions with different velocities.
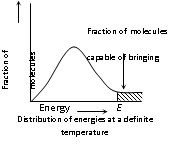
(iv) For the collision to be effective enough, under given are the two barriers which are to be cleared,
(a) Energy barrier : The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy.
· In graph given to us E corresponds to minimum or the threshold energy for effective collision.
· There is an energy barrier for each reaction. The reacting species should be provided sufficient energy to cross energy barrier.
(b) Orientation barrier : The colliding molecules should also have proper orientation so that the old bonds may break and new bonds are formed. For example, 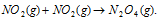 during the reaction, products are formed at the time when colliding molecules have proper orientation at the time of collisions. These are usually known as as effective collisions.
during the reaction, products are formed at the time when colliding molecules have proper orientation at the time of collisions. These are usually known as as effective collisions.
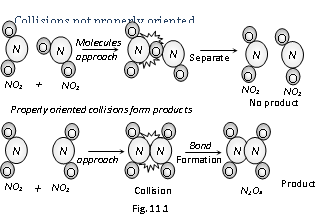
(v) Therefore, the main points of the collision theory are given as follows,
(a) If the reaction is to take place there should be collisions among the reacting species.
(b) The particular fraction of total the number of collisions is effective in forming products.
(c) For effective collisions, molecules should posses' sufficient energy as well as orientation.
(vi) The fraction of effective collisions, under the normal conditions can vary from nearly zero to about one for ordinary reactions. Hence, the rate of reaction is proportional to :
(a) The number of collisions per unit volume per second (Collision frequency, Z) among the reacting species
(b) The fraction of effective collisions (Properly oriented and possessing sufficient energy), f that is Rate=-dx/dt=f*Z
Here f is the fraction of effective collision and Z is the collision frequency of it.
(vii) The physical meaning of activation energy is the minimum relative kinetic energy which the reactant molecules must possess for changing into the products molecules during their collision. Which means that the fraction of successful collision is equal to 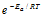 termed as Boltzmann factor.
termed as Boltzmann factor.
(viii) It can be noted that besides the need of sufficient energy, the molecules should be properly oriented in space also for the collision to be successful. Hence, if ZAB is collision frequency, P is the orientation factor (or Steric factor) then, 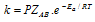 . When we compare this equation with the Arrhenius equation
. When we compare this equation with the Arrhenius equation 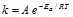 .
.
We already know that pre-exponential form 'A' in the Arrhenius equation is, A=PZAB.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Theories of reaction rate-Collision theory questions? Theories of reaction rate-Collision theory topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Theories of reaction rate-Collision theory related problems. We provide step by step Theories of reaction rate-Collision theory question's answers with 100% plagiarism free content. We prepare quality content and notes for Theories of reaction rate-Collision theory topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours