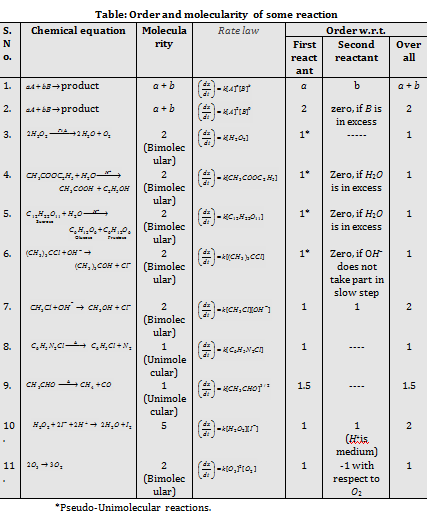
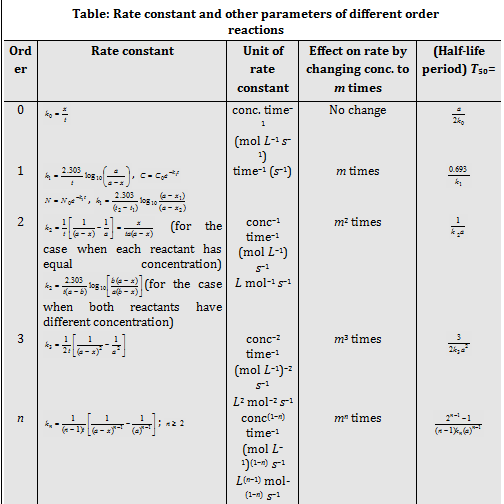
Rate law the Molecularity and Order of a reaction
The Molecularity is sum of the number of molecules of reactants involved in the balanced chemical equation. The Molecularity of a whole reaction has no importance and overall kinetics of the reaction depends upon the rate determining step. The slowest step is rate-determining step. This was given by Van't Hoff.
Example :
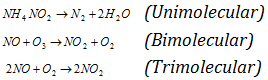
Total number of the molecules or atoms whose concentration determines the rate of reaction is called as order of reaction.
The Order of reaction = Sum of the exponents of the concentration terms in rate law
For the reaction xA+yB→Products
The rate law is Rate = [A]x[B]y
Then the overall order of the reaction. n=x+y
Here x and y are orders with respect to the individual reactants.
· If the reaction is in form of reaction mechanism then the order is determined by slowest step of mechanism.
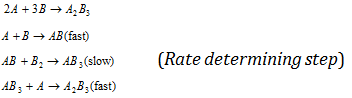
(Here, the overall order of the reaction is equal to two.)
· The Molecularity of a reaction is derived from mechanism of the given reaction. The Molecularity cannot be greater than three because more than three molecules might not mutually collide with each other.
· The Molecularity of a reaction can't be zero, negative or fractional. Order of the reaction can be zero, negative, positive or in fraction and greater than three. The Infinite and imaginary values are not possible.
· It one of the reactants is present in the excess, the second order reaction conforms to the first order and is called as pesudo unimolecular reaction.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Rate law - Molecularity and Order of a reaction questions? Rate law - Molecularity and Order of a reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Rate law - Molecularity and Order of a reaction related problems. We provide step by step Rate law - Molecularity and Order of a reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Rate law - Molecularity and Order of a reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours