Protection of colloids and Gold number
- Lyophilic solutions are much more stable than the lyophobic sols.
- Lyophobic solutions can be simply coagulated by the addition of small quantity of an electrolyte.
- When a lyophilic sol is added to any lyophobic solution, which becomes less sensitive towards electrolytes. Therefore, lyophilic colloids can prevent the coagulation of any lyophobic sol.
"The phenomenon of preventing the coagulation of a lyophobic sol due to the addition of some lyophilic colloid is called solution protection or protection of the colloids."
- The protecting power of different protective (lyophilic) colloids is different. The efficiency of any of the protective colloid is expressed in terms of gold number.
Gold number : Zsigmondy introduced a term called gold number to describe the protective power of different colloids. This can be defined as, "weight of the dried protective agent in milligrams, this when added to the 10 ml of a standard gold sol (0.0053 to 0.0058%) is just sufficient to prevent a colour change from red to blue on the addition of 1 ml of 10 % sodium chloride solution, is equivalent to the gold number of that protective colloid."
Hence, smaller is the gold number; higher is the protective action of the protective agent.
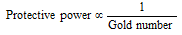
Table: Gold numbers of some hydrophilic substances
|
Hydrophilic substance
|
Gold number
|
Hydrophilic substance
|
Gold number
|
|
Gelatin
|
0.005 - 0.01
|
Sodium oleate
|
0.4 - 1.0
|
|
Sodium caseinate
|
0.01
|
Gum tragacanth
|
2
|
|
Hamoglobin
|
0.03 - 0.07
|
Potato starch
|
25
|
|
Gum arabic
|
0.15 - 0.25
|
|
|
Congo rubin number : Ostwald introduced congo rubin number to account for protective nature of colloids. It can be defined as the amount of protective colloid in milligrams which prevents colour alter in the 100 ml of 0.01 % congo rubin dye to which 0.16 g equivalent of the KCl is added.
Mechanism of sol protection
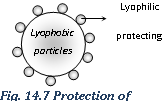
(i) The actual mechanism of sol protection is very complex. Although it may be due to the adsorption of the protective colloid on the lyophobic sol particles, followed by its solvation. Therefore it stabilises the sol via solvation effects.
(ii) Solvation effects contribute much towards the stability of lyophilic systems. For instance, gelatin has a sufficiently strong affinity for water. It is only due to the solvation effects that even the addition of electrolytes in small amounts does not cause any flocculation of hydrophilic sols. Although at higher concentration, precipitation occurs. This process is called salting out.
(iii) Salting out efficiency of the electrolyte depends upon the tendency of its constituents ions to get hydrated that is the tendency to squeeze out water initially fied up with the colloidal particle.
(iv) The anions and the cations can be arranged in decreasing order of the salting out power; this type of arrangement is called as lyotropic series.
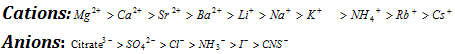
Ammonium sulphate, due to its very high solubility in water, is often used for precipitating proteins from aqueous solutions.
(v) The precipitation of lyophilic colloids can also be affected by the addition of organic solvents of non-electrolytes. For instance, the addition of acetone or alcohol to aqueous gelatin solution causes precipitation of gelatin. The addition of the petroleum ether to a solution of rubber in benzene causes the precipitation of rubber.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Protection of colloids and Gold number questions? Protection of colloids and Gold number topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Protection of colloids and Gold number related problems. We provide step by step Protection of colloids and Gold number question's answers with 100% plagiarism free content. We prepare quality content and notes for Protection of colloids and Gold number topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.=
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours