Potassium Dichromate, (K2Cr2O7)
The K2Cr2O7 (potassium dichromate) is one of the most important compounds of chromium, and as well among the dichromates. In this type of compound Cr is in hexavalent (+6) state.
Preparation: It can be simply prepared through any of the below stated techniques,
- By potassium chromate: in the first technique Potassium dichromate can be acquired by adding the measured amount of sulphuric acid to the potassium chromate's saturated solution.
Technique is described in the equation below:
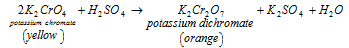
K2Cr2O7 Crystals can be acquired through concentrating the solution and crystallisation.
- Manufacture from chromite ore: in the second technique K2Cr2O7 is usually made from chromite ore (FeCr2O4). The procedure includes the steps that are described below:
a) Preparation of sodium chromate: in this first step the powdered chromite ore is mixed with soda ash and quicklime very finely. After that Mixture is roasted in the reverberatory furnace in existence of air. Yellow mass due to the formation of the sodium chromate is acquired.
The equation for Produce from chromite ore technique is as follow:
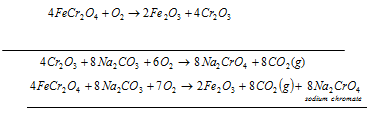
With water yellow mass is extracted, and after that it is filtered. The filtrate has sodium chromate.
The reaction can as well be performed by taking in use NaOH instead of Na2CO3. In this type of case the reaction is like,
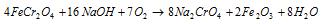
b) Conversion of the chromate into dichromate: in this second step the sodium chromate solution acquired in step (a) is treated along with the concentrated sulphuric acid while it is transformed into the sodium dichromate. This is explained in the equation below:
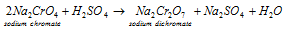
The less soluble sodium sulphate that is Na2SO4, on concentration. 10H2O crystallizes out. This is filtered hot and let cool while sodium dichromate, Na2Cr2O7.2H2O, obtains separates on standing.
c) Concentration of the sodium dichromate to potassium dichromate: in this third step hot concentrated solution of sodium dichromate is treated along with an amount of potassium chloride that is calculated before. While the potassium dichromate that is less soluble crystallizes out on cooling.
d)
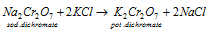
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Potassium Dichromate questions? Potassium Dichromate topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Potassium Dichromate related problems. We provide step by step Potassium Dichromate question's answers with 100% plagiarism free content. We prepare quality content and notes for Potassium Dichromate topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours