Ideal and Non-Ideal solution
Table: Ideal and non-ideal solutions
|
Ideal solutions
|
Non-ideal solutions
|
|
Positive deviation from Raoult's law
|
Negative deviation from Raoult's law
|
|
1. Obey Raoult's law at every range of concentration.
|
1. Do not obey Raoult's law.
|
1. Do not obey Raoult's law.
|
|
2. ΔHmix = 0; neither heat is evolved nor absorbed during dissolution.
|
2. ΔHmix > 0 Endothermic dissolution; heat is absorbed.
|
2. ΔHmix < 0 Exothermic dissolution; heat is evolved.
|
|
3. ΔVmix = 0; total volume of solution is equal to sum of volumes of the components.
|
3. ΔVmix > 0 Volume is increased after dissolution.
|
3. ΔVmix < 0 Volume is decreased during dissolution.
|
|
4. 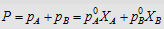 i.e., i.e.,
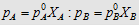
|
4. 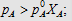 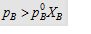
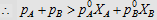
|
4. 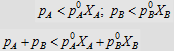
|
|
5. A-A, A-B, B-B interactions should be same, i.e., 'A' and 'B' are identical in shape, size and character.
|
5. A-B attractive force should be weaker than A-A and B-B attractive forces. 'A' and 'B' have different shape, size and character.
|
5. A-B attractive force should be greater than A-A and B-B attractive forces. 'A' and 'B' have different shape, size and character.
|
|
6. Escaping tendency of 'A' and 'B' should be same in pure liquids and in the solution.
|
6. 'A' and 'B' escape easily showing higher vapour pressure than the expected value.
|
6. Escaping tendency of both components 'A' and 'B' is lowered showing lower vapour pressure than expected ideally.
|
|
Examples:
Dilute solutions;
benzene + toluene:
n-hexane + n-heptane;
chlorobenzene + bromobenzene;
ethyl bromide + ethyl iodide;
n-butyl chloride + n-butyl bromide
|
Examples:
Acetone +ethanol
acetone +CS2:
water + methanol;
water + ethanol;
CCl4 + toluene;
CCl4 + CHCl3;
acetone + benzene;
CCl4+CH3OH;
cyclohexane + ethanol
|
Examples:
Acetone + aniline;
acetone + chloroform;
CH3OH+CH3COOH;
H2O+HNO3
chloroform + diethyl ether;
water + HCl;
acetic acid + pyridine;
chloroform + benzene
|
| |
|
|
|
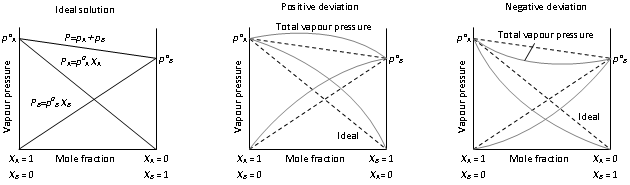
Graphical representation of ideal and non-ideal solutions
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ideal and Non-Ideal Solutions questions? Ideal and Non-Ideal Solutions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ideal and Non-Ideal Solutions related problems. We provide step by step Ideal and Non-Ideal Solutions question's answers with 100% plagiarism free content. We prepare quality content and notes for Ideal and Non-Ideal Solutions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours