The depression or decrease in the freezing point of the solvent (Cryoscopy)
The Freezing point is the temperature at which liquid and solid states of the substance are in equilibrium with each other or it can be defined as the temperature at which the liquid and the solid states of the substance have the same vapour pressure. It is observed that freezing point of the solution is always less than the freezing point of pure solvent. Therefore the freezing point of sea water is low than that of pure water. The depression in freezing point ΔT or ΔTf of a solvent is the difference in the freezing point of the pure solvent (Ts) and the solution (Tsol).
ΔTs - ΔTsol = Δ Tf or ΔT
NaCl or CaCl2 (anhydrous) are used to clear the snow on the roads. They depress the freezing point of the water and hence reduce the temperature of the formation of the ice.
Depression in the freezing point is determined by the Beckmann's method and the Rast's camphor method. Study of depression in the freezing point of a liquid in which a non-volatile solute is dissolved in it is termed as cryoscopy.
Important relations concerning depression in freezing point.
(1) Depression in freezing point is directly proportional to the lowering of vapour pressure. 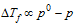
(2) 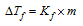
Where Kf = molal depression constant or cryoscopic constant; m = Molality of the solution (i.e., no. of moles of solute per 1000g of the solvent); ΔTf = Depression in freezing point
(3) 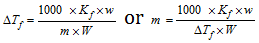
where Kf is molal depression constant and defined as the depression in freezing point produced when 1 mole of the solute is dissolved in 1 kg of the solvent. w and W are the weights of solute and solvent and m is the molecular weight of the solute.
(4) 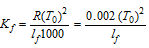
where T0 = Normal freezing point of the solvent; Lf Latent heat of fusion/g of solvent; Kf for water is 1.86 deg-kg mol-1
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Depression in freezing point or Cryoscopy questions? Depression in freezing point or Cryoscopy topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Depression in freezing point or Cryoscopy related problems. We provide step by step Depression in freezing point or Cryoscopy question's answers with 100% plagiarism free content. We prepare quality content and notes for Depression in freezing point or Cryoscopy topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours