Pyramidal inversion:
As amines are tetrahedral, they are chiral if they comprise three different substituents. Though, it is not possible to separate the enantiomers of a chiral amine as amines can easily go through pyramidal inversion - a process that inter converts the enantiomers. The inversion includes a change of hybridization in which the nitrogen becomes sp2 hybridized rather than sp3 hybridized.
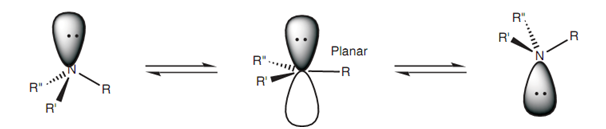
Figure: Pyramidal inversion.
As a result, the molecule turns into planar and the lone pair of electrons takes place a p orbital. One time the hybridization reverts back to sp3, the molecule can either revert back to the original shape of it or invert.
Even though the enantiomers of chiral amines cannot be separated, such types of amines can be alkylated to make quaternary ammonium salts in which the enantiomers can be separated. One time the lone pair of electrons is locked up in σ (sigma) bond, pyramidal inversion becomes not possible and the enantiomers can no longer interconvert.