Rate Theory:
This concept actually visualizes the role of different kinetic variables on zone broadening. The migration of the solute down the column takes place as a result of several mass transfers and there is some time which these mass transfers take place. The kinetic effects depend upon the length of time; the mobile phase spends in contact with the stationary phase. That, in turn, depends upon the flow rate of the mobile phase. For this purpose the studies on the efficiency of the column are carried out by determining HETP (H) as a function of mobile phase velocity (u). The shapes of two such plots, one for the liquid chromatography and the other for gas chromatography are shown in Figure.
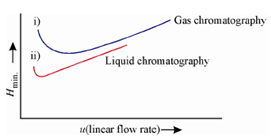
Figure: Dependence of plate height on the flow rate of mobile phase i) liquid chromatography ii) gas chromatography
In both the cases, there is a Hmin at a low flow rate. The minima for liquid chromatography occur generally at flow rates well below those for gas chromatography. In addition, in liquid chromatography, a flow rates for Hmin. is frequent so low in which they are not observed under normal working conditions. In gas chromatography, the separations are achieved in shorter times than those attained in liquid chromatography.