Deviations from Beer-Lambert's Law:
As per the Beer's law elaborates above, there is a direct proportionality among the absorbance and concentration. The plot of absorbance versus concentration is expected to be a straight line passing by origin. Thus, this is not always true; there are certain limitations. A law does not carry for all species under every condition. Several a times instead of a straight line, a curvature within the plot might be observed as shown in Figure. An upward curvature, curve (a), is called as positive deviation and the downward curvature, curve(c), since negative deviation.
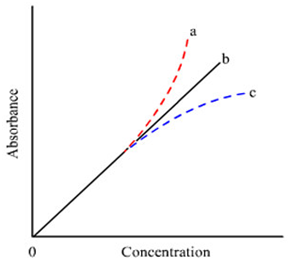
Figure: Beer-Lambert law plots; the curvatures show deviations from the law
A few of the factors responsible for the deviation from Beer's law are as follows.