Standard Cell:
In the previous sub-section you have seen that there is a need of standard source of potential for the measurement of potential of unknown electrolytic cell. The mostcommonly used standard cell in potentiometry is the Weston Cell (Weston cadmium cell) which is depicted in Figure and can be represented by
Cd(Hg)|CdSO4.8/3 H2O (s)|| Hg2SO4(s)|Hg ... (2.13)
where Cd(Hg) represents the solution of cadmium in mercury. The two half-cell reactions occurring in this cell are
Cd (Hg) → Cd 2++ Hg (1) + 2e ... (2.14)
Hg 2++ 2e → 2Hg(1) ..... (2.15)
Cell reaction: Cd + Hg2SO4 + 8/3 H2O ↔ CdSO4.8/3 H2O + 2Hg
The potential of this Weston Cell is 1.0183 V at 20oC. It is temperature dependent and is given by 1.0183 - 0.0000406 (t - 200) at any other temperature t. Actually, in practice there are small variations in the emf of Weston Cells supplied by different manufacturers and it is essential to use the exact value quoted for the cell. While using it or preferabl y the value obtained by calibrations of the cell in use against a Weston Cell of known emf which is always kept reserved only for calibrations purposes. At no time current be drawn for longer times from a Weston Cell. A typical design of Weston cadmium cell is shown in Figure.
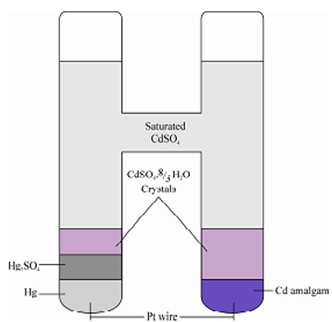
Figure: The Weston cadmium cell
Potentiometric measurement using potentiometer discussed above is quite suitable if galvanic cell having internal resistance less than 103 ?. Most of cells containing membrane electrode like glass electrode have high internal resistance (>109 ?). To measure potential of such high resistance cell accurately, it is necessary that voltmeter device have resistance that is several order of magnitude greater than the resistance of the cell being measured. If the meter resistance is too low, the current is drawn from the cell, this result in lowering its output potential, thus creating a negative loading error. Because of this, digital voltmeters (dvm) are preferred for the measurement of potential as these offers the great advantage of having large internal resistance (1011 to 1012 ?). Digital voltmeters are marketed as pH meters or ion meters.