Electrochemical energy
In electricity, one significant form of potential energy exists in atoms and molecules of some chemicals under the special conditions. Early in history of electrical science, laboratory physicists found that when the metals came into contact with some chemical solutions, voltages appeared between the pieces of metal. These were the 1st electrochemical cells.
A piece of lead and a piece of lead dioxide immersed in the acid solution will show the persistent voltage. This is detected by connecting a galvanometer between the pieces of metal. A resistor of about 1,000 ohms should always be used in the series with galvanometer in experiments of this kind; connecting galvanometer directly will cause large amount of current to flow, possibly damaging te galvanometer and resulting in the acid to boil.
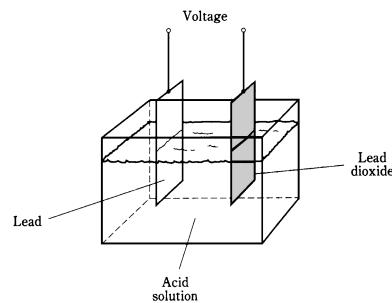
Figure-- Construction of a lead-acid electrochemical cell.
The chemicals and metal have an inherent ability to generate a constant exchange of charge carriers. If galvanometer and resistor are left connected between the 2 pieces of metal for a long time, the current will decrease gradually, and the electrodes will become coated. The acid will change, also, the chemical energy, a form of potential energy in acid, will run out. All potential energy in the acid will have been turned into kinetic electrical energy as the current in wire and galvanometer. In turn, this current will have heated resistor (another form of kinetic energy), and escaped into the air and space.