Q. Compounds of Group 16 Elements?
We will now discuss some compounds like the hydrides, halides and oxides of the Group 16 elements.
All elements of this group form simple volatile binary hydrides of the type H2X (X = 0. S etc.). The simple hydride of oxygen is H2O or water. The other known hydride of oxygen is H202 hydrogen peroxide. Sulphur forms the most extensive series of catenated hydrides such as H2S, H2S2, H2S2 H2 S2 etc. Hydrides of sulphur, other than H2S, are all yellow oils which readily decompose into H2S and free sulphur. Selenium, tellurium and polonium form H2, Se, H2Te and H2Po, respectively.
The reducing power of hydrides increases whereas their thermal stability decreases in the order H2O; H2S; H2, Se; H2, Te; H2Po. In fact H2Se and H2Te are better reducing agents than hydrogen.
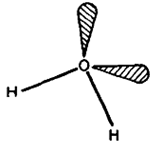
The hydride molecules are angular in shape. The bond angles in H2O, H2S, H2Se and H2Te being 104.50. 920.910 and 89.0°, respectively. In case of H2O, oxygen is supposed to be sp7 hybridised with two lone pairs occupying two positions on the tetrahedron, Fig. The distortion from the tetrahedral angle (109028') is supposed to be due to stronger repulsion between, lone pairs of electrons compressing the bond angle. In the case of other hydrides, bond angles are close to 900 suggesting that almost pure p -orbitals are inv6lved in bonding to hydrogen.