Reference no: EM13140
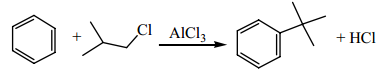
Question:1 Show all the steps in the mechanism for the following reaction.
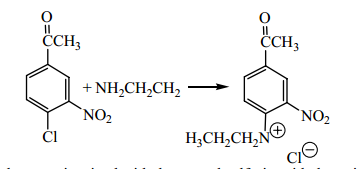
Question: 2 Show all the steps in the mechanism for the following reaction. Explain why the acetyl group accelerates the reaction
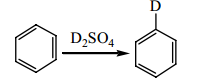
Question 3: When benzene is mixed with deuterated sulfuric acid, deuterium is slowly incorporated onto the ring. Show the mechanism for this reaction and explain how this relates the sulfonation of benzene .
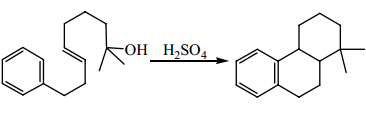
Question 4: Show the steps in the mechanism for the following reaction:
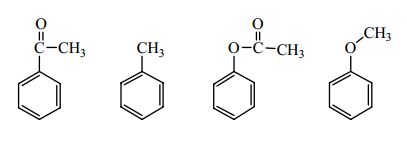
Question 5 : Arrange the following compounds in order if increasing rate of reaction towards a nitration reaction.
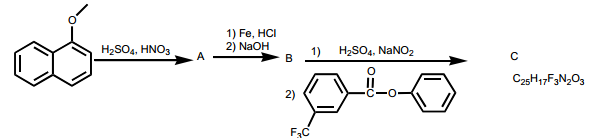
Question 6 : What are products of the following reactions? For any electrophilic aromatic substitution reaction, draw only the major monosubstituted product.
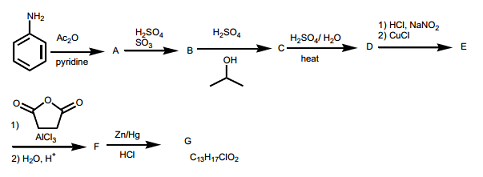
Question 7: What are products of the following reactions? For any electrophilic aromatic substitution reaction, draw only the major monosubstituted product.
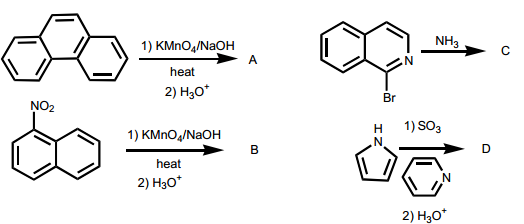
Question: 8 - > What are products of the following reactions?
|
Individual assignment: two models handout and rubric
: Individual Assignment : Two Models Handout and Rubric, This paper will allow you to understand and evaluate two vastly different organizational models and to effectively communicate their differences.
|
|
In crisis and reform
: In Crisis and Reform: Current Issues in American Punishment (p. 126), Alexis Durham writes,Please write a reaction paper (4-6 pages in length) to the quote in which you explore global correctional objectives
|
|
Environmental economics
: There are three alternative plans that indicate the benefits and costs associated with the construction of a Manitoba hazardous waste facility (see table on next page).
|
|
Steps in the mechanism for the following reaction
: Show all the steps in the mechanism for the following reaction, When benzene is mixed with deuterated sulfuric acid, deuterium is slowly incorporated onto the ring. Show the mechanism for this reaction and explain how this relates the sulfonation of ..
|
|
Corporate culture:what makes an organization
: This assignment is highlight the integrating the concept of organizational culture and organizational values in the business organisations.
|
|
Disorder paper: schizophrenia
: Schizophrenia does not really have just one single cause. It is a possibility that this disorder could be inherited but not all doctors are sure.
|
|
A business plan: gift shop
: A business plan for a gift shop contains so many constraints and aspects to be taken into consideration before start up with something.
|
|
A project on: mumbai rescued victim centre project
: This paper is a comprehensive macro-management presentation of the proposed Mumbai Rescued Victims Center (MRVC), which is modeled after the Nampa Family Justice Center.
|